About Ecolab’s Bioquell
In hospitals, pharmaceutical manufacturing plants, labs and cleanrooms, reducing the risk of microbial contamination is vital. You need a reliable way to bio-decontaminate your facilities and equipment. Bioquell solutions empower you to improve product and patient safety, meet regulatory and compliance requirements, and reach maximum operational efficiency.
- Effective
Bioquell bio-decontamination equipment uses 35% aqueous hydrogen peroxide to produce hydrogen peroxide vapour, which achieves a 6-log sporicidal kill on exposed, non-porous surfaces. This process kills a wide range of microorganisms including bacteria, viruses, fungi and spores.
- Trusted
Bioquell is used in healthcare and life science facilities around the world to bio-decontaminate operating rooms, patient rooms, pharmaceutical manufacturing centres, and more.
- Regulatory Compliance
Ecolab supports Bioquell technology with professional validation, maintenance and customer service. Bio-decontamination with Bioquell hydrogen peroxide vapour meets or exceeds regulatory standards around the world, including the European Biocidal Product Regulation (BPR).
The Hydrogen Peroxide Vapour
Bio-decontamination Process
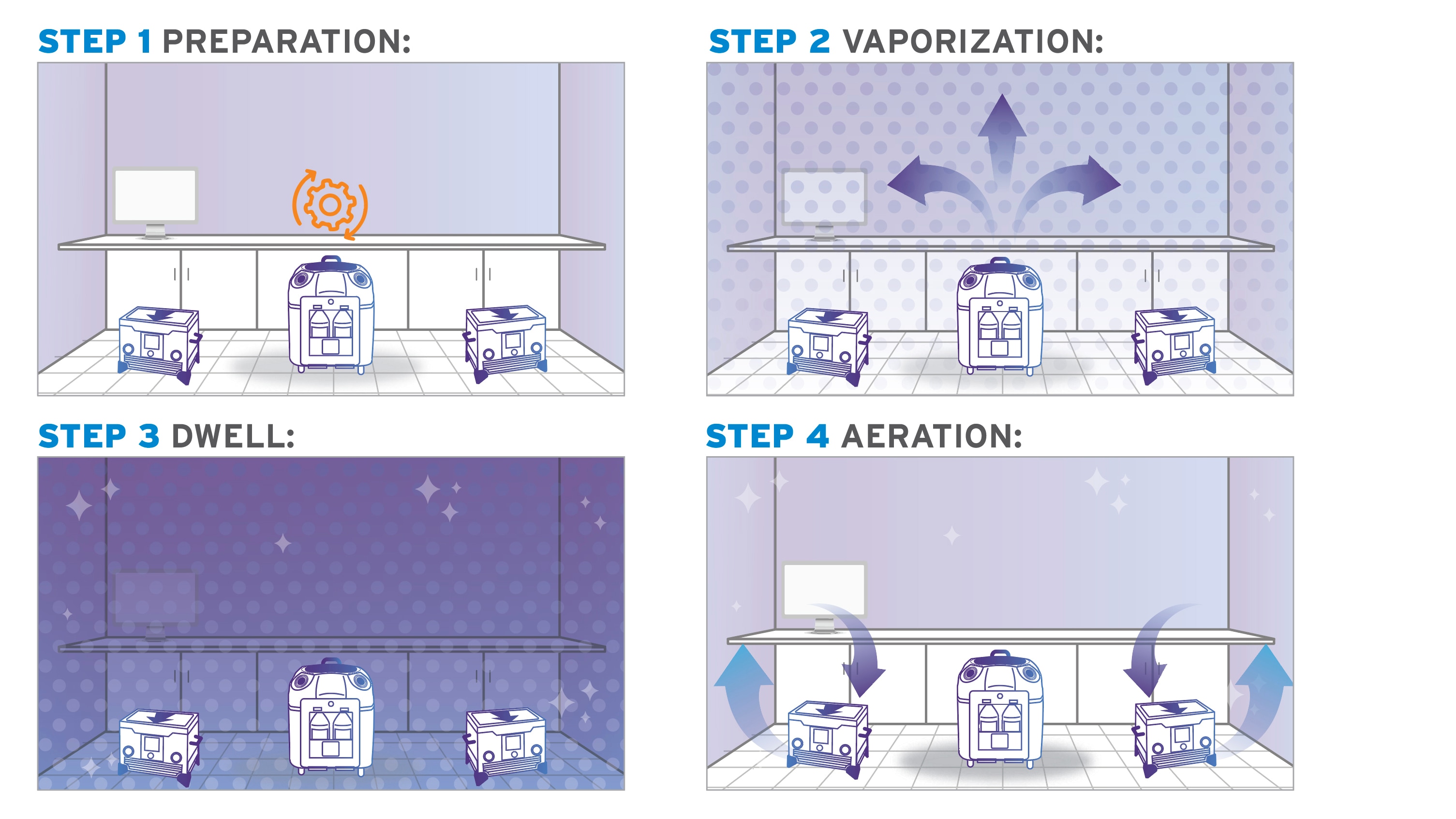
1: Preparation
Prep the room for bio-decontamination: seal vents, and open any cabinets and drawers that require treatment. Seal the door after you exit, then turn on the Bioquell equipment.
2: Vaporization
The system emits the vapour, filling the enclosed area. It deposits a micro-condensation on exposed surfaces, including complex shapes and crevices.
3: Activation Period
Once vaporization is complete, a waiting period allows the hydrogen peroxide time to kill microorganisms on surfaces.
4: Aeration
Lastly, your HVAC system and/or Bioquell aeration units convert the H2O2 into water vapour and oxygen. There are no additional steps to remove residue—the room is ready now ready for use.

Bio-decontamination for Biopharmaceutical Manufacturing
Whether you work in cell and gene therapy or vaccine development, it's important to have a solid bio-decontamination solution in place.

Hydrogen Peroxide Vapour Systems and Services
If you’re seeking a reliable way to kill bio-contaminants in your lab or manufacturing facility, look no further than Bioquell equipment and services.

Validation
To meet regulatory requirements, you may need to provide proof that your bio-decontamination solution works. Ecolab provides validation documentation to support your needs.
Related Bioquell Bio-decontamination
Equipment and Services
Learn more about how Ecolab’s Bio-decontamination Equipment and Services solutions can help your facility produce safe, compliant products that exceed standards, measurably improve operational efficiency and help achieve sustainability goals.
Life Sciences Insights
Learn more about Ecolab’s role in the Life Science Industry.


